Call Us: 980-236-8605
Covid-19 Rapid Antigen Test
By CareStart™

CareStart is intended for use by medical professionals
- Lateral Flow Test
- Results at 10 minutes
- Anterior Nares: 87.2% sensitivity, 100% specificity Nasopharyngeal: 93.8 % sensitivity, 99.3% specificity
- No special equipment required
Limited supply
Benefits
fast and easy
You can self-test anywhere in 10 minutes
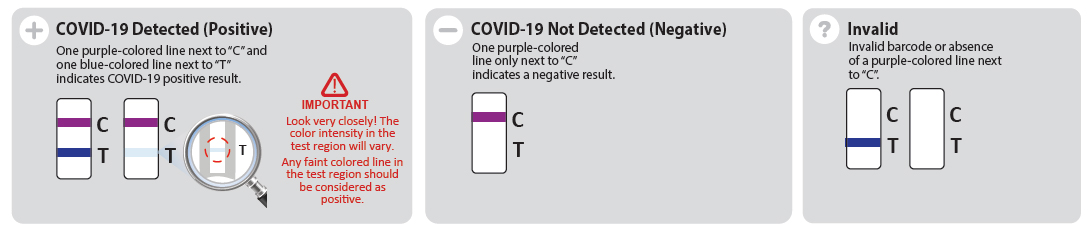
Easy to interpret
Interpret the results is very easy using mobile application
Very accurate
Qualitatively detect the SARS-CoV-2 nucleocapsid protein
Limited supply
What Our customers say
real reviews verifiable on google reviews




Limited supply
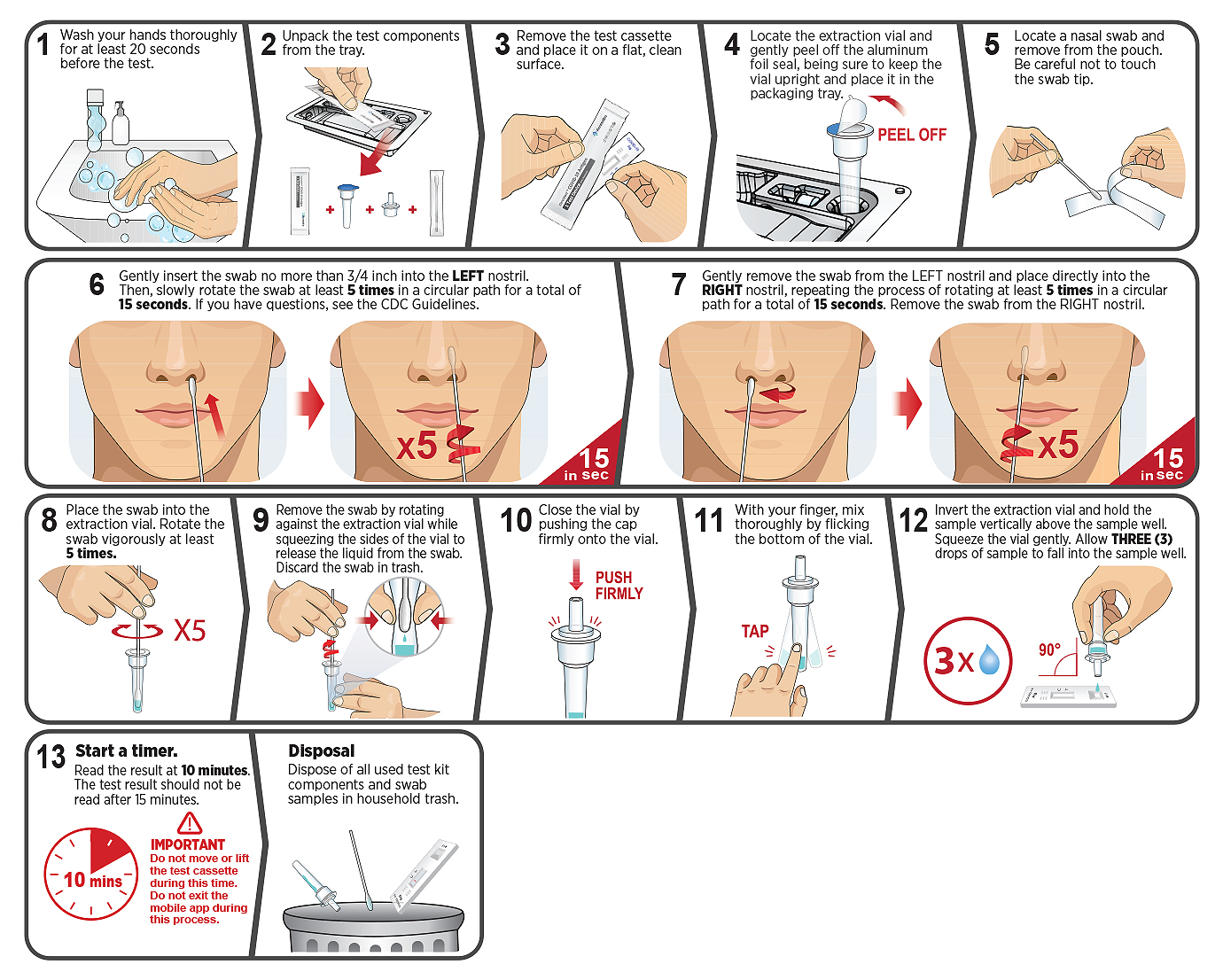
Self Test for SARS-CoV-2 Antigen Detection
The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2.
Under FDA’s EUA, CareStart™ COVID-19 Antigen Home Test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older with symptoms of COVID-19 within the first 7 days of symptom onset.
This test is also authorized for non-prescription home use with adult-collected nasal (nares) swab samples from individuals aged 2 years or older with symptoms of COVID-19 within the first 7 days of symptom onset.
This test is also authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older, or adult-collected anterior nasal (nares) swab samples from individuals aged 2 years or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
For in vitro diagnostic use only. For prescription use only.
Limited supply
Frequently asked questions
Limited supply
Disclaimer:
As of November 22, 2021, CareStart™ COVID-19 Antigen Home Test is authorized to use with individual anterior nasal swab specimens from individuals age 14 years and older (self-collected), or 2 years and older (collected with adult assistance) for non-prescription home use by FDA under the EUA (EUA210314/S002). The authorized labeling has been changed with the new indication (Quick Reference Instructions (QRI) and Fact Sheet for Individuals, Instructions for Use (IFU) for Healthcare Providers (HCP), Fact Sheet for HCP, and kit package); however, CareStart™ COVID-19 Antigen Home Test will continue to be distributed with the labeling previously authorized per EUA210314/S001 on the FDA’s enforcement discretion in efforts to increase the testing accessibility for COVID-19. Since the differences between the CareStart™ COVID-19 Antigen Home Test kit previously authorized (EUA210314/S001) and currently authorized (EUA210314/S002) are limited to the authorized labeling, you may use the CareStart™ COVID-19 Antigen Home Test kit with the new indication for use (described above).
Please fill out the below form and we will get back you as soon as we can.
Contact Us For Information
MORE INFORMATION ON HOW TO ORDER.
Please provide your information, and we will contact you within one business day.
Copyright © 2022 CoviLab Charlotte | Charlotte COVID-19 Testing Center
Powered by CoviLab Charlotte